In addition to the normal criteria of installation and verification operations non-exhaustive list.
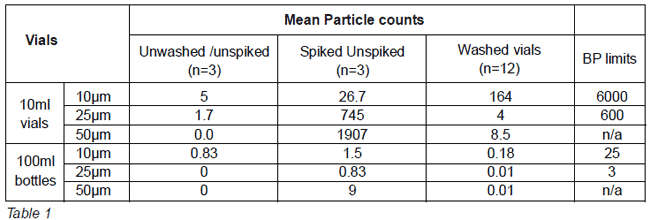
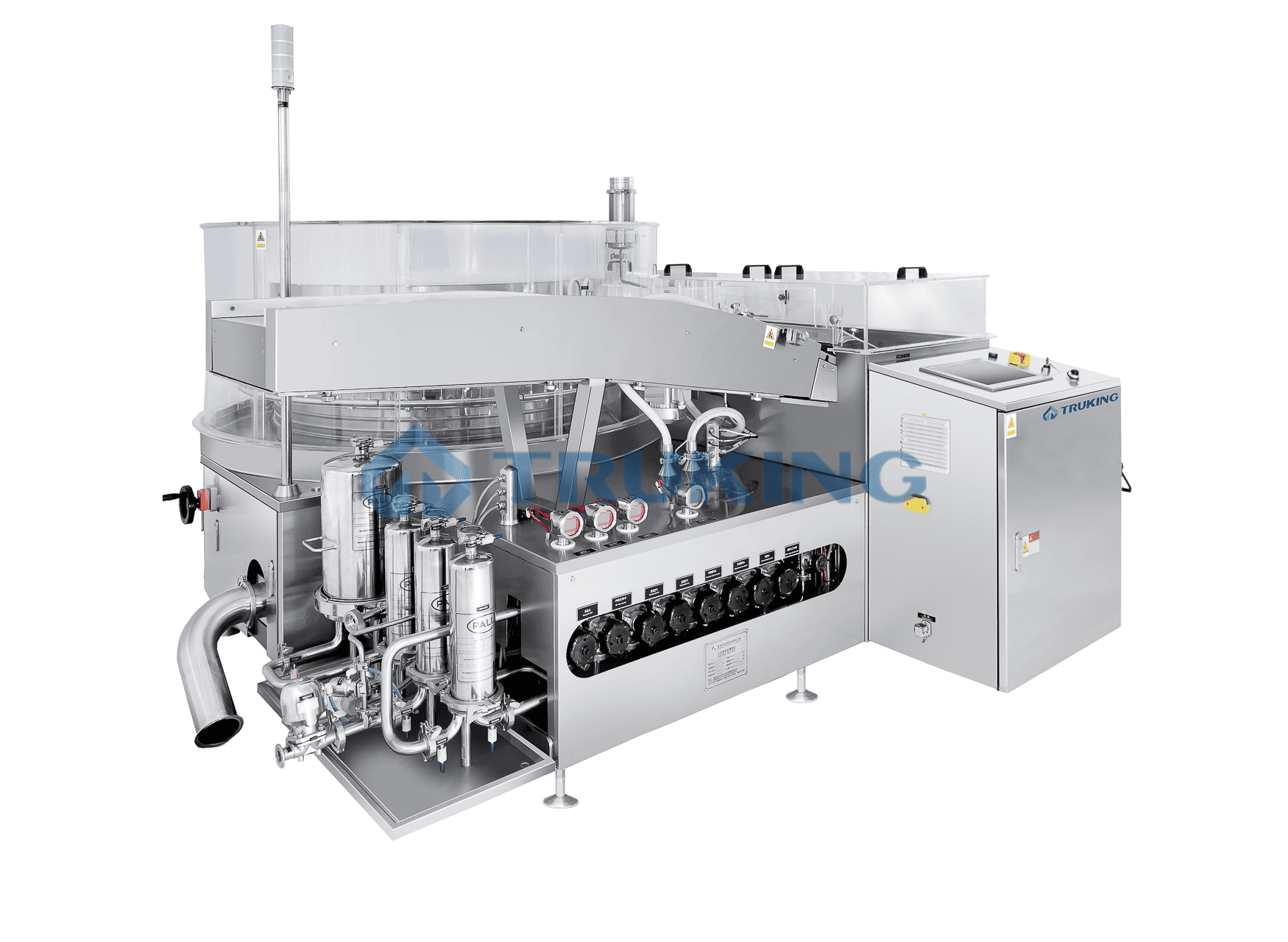
Vial washing machine validation protocol. VIAL WASHING MACHINE PQ PROTOCOL. At this point a particulate analysis using a particulate counter is performed testing for particulates 10-25µm. 4 Liquid forms a vortex imparts movement of insoluble part 5 Vial stops vortex collapses image is projected with variation in.
As the internal surface of the vial is product-direct contact a certain quantity of each contaminant will be placed into the vials and washed in the vial washer. Accords validated washing protocols utilise industrial equipment that subjects each vial to a double rinse with purified water and then dried with compressed air. It should be done three times for each vial size.
The vials are then washed in the vial washer Once washed the vials are filled and rinsed with purified water. The machine contains a light-transmission double-check system for detecting particles in ampoules. These spiked vials are analyzed separately.
To be performed at the time of relocation or Re-qualification. Particulate matter contaminants such. 18 1 Static Division system 2 Divides photo-detection system into individual parts detection is done from base of ampoule.
Some drug manufacturers only perform Installation Qualification IQ and Operational Qualification OQ of the machine as no regulatory requirements clearly state that the performance of the vial washer should be qualified. To be performed after the completion and authorization of Operational Qualification. A written report summarizing results and conclusions should be recorded prepared and.
This stage requires a validation strategy. These vials should be placed at the initial middle and end of the washing session. A vial washer is a relatively simple machine commonly used to clean containers during the manufacture of dosage form drugs.